

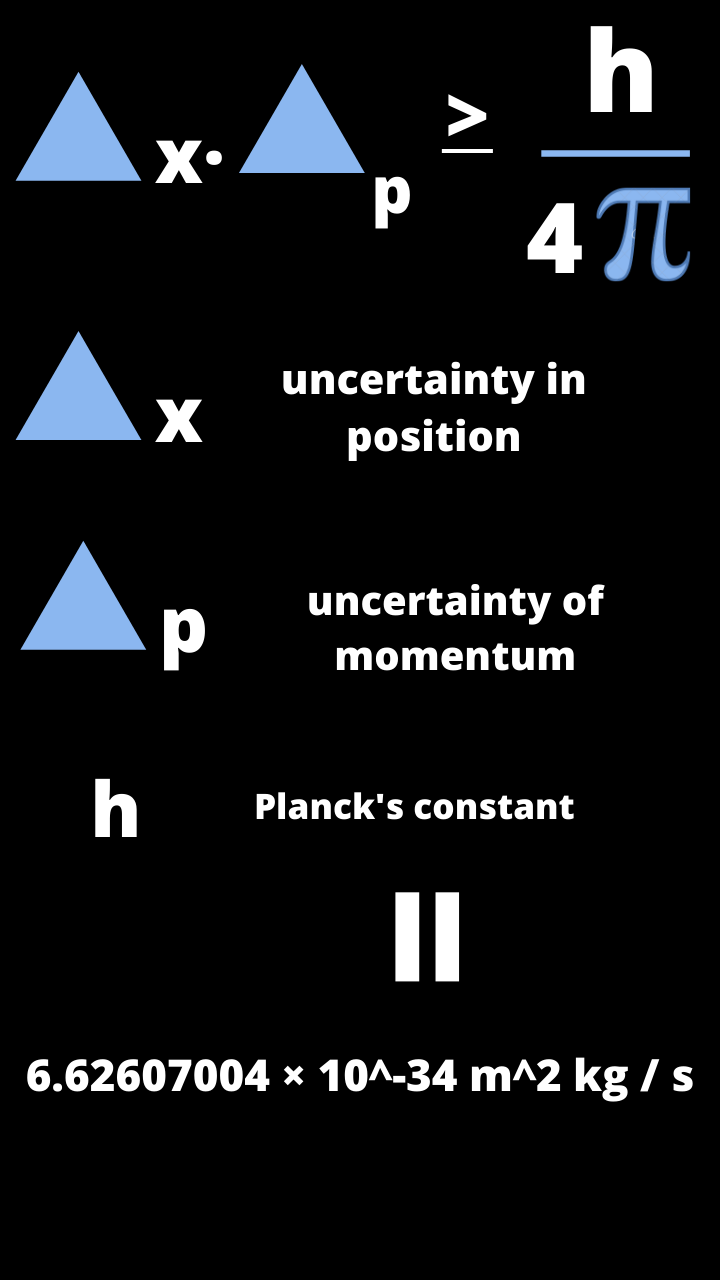
Learning about the electron's position would create uncertainty in its velocity and the act of measurement would produce the uncertainty needed to satisfy the principle. That would reveal its position, but it would also impart energy to the electron, causing it to move. To take the picture, a scientist might bounce a light particle off the electron's surface. His most well-known thought experiment involved photographing an electron. Heisenberg sometimes explained the uncertainty principle as a problem of making measurements. The limit is expressed as a simple equation that is straightforward to prove mathematically. For example, the more precisely one knows a particle's position, the less one can know about its momentum, and vice versa. Simply put, the principle states that there is a fundamental limit to what one can know about a quantum system. The study overthrows a common classroom explanation of why the quantum world appears so fuzzy, but the fundamental limit to what is knowable at the smallest scales remains unchanged.Īt the foundation of quantum mechanics is the Heisenberg uncertainty principle. A new experiment shows that measuring a quantum system does not necessarily introduce uncertainty. Contrary to what many students are taught, quantum uncertainty may not always be in the eye of the beholder.


 0 kommentar(er)
0 kommentar(er)
